Malignant melanoma is a potentially fatal form of skin cancer, with a strong capacity for invasion and metastasis, and high rates of recurrence and mortality. The median survival following the onset of distant melanoma metastasis is just 6-9 months and the five year survival rate is<5%. Melanoma progression and metastasis is necessarily a complex multistep process, as a cancer cell must acquire the ability to survive under anchorage independent conditions, invade through the surrounding stroma and migrate, intravasate into the vascular system; extravasate and subsequently endure a disadvantageous distant environment, adhere to the local tissue, and proliferate.
Matrix metalloproteinases (MMPs), which are the most important factor secreted by tumor cells, stromal fibroblasts, or infiltrating inflammatory cells in the tumor microenvironment, have been strongly implicated in multiple stages of invasive and metastatic progression of tumor cells. Furthermore, MMP-9 is important for tumor angiogenesis by enhancing the availability of vascular endothelial cell growth factor (VEGF) in malignant tumors. The interaction of VEGF with its cognate receptor VEGFR on the surface of endothelial cells promotes the recruitment and proliferation of endothelial cells via the activation of PI3K/Akt and Ras/Raf/MEK/ERK signaling. In the majority of cancer patients, by the time of diagnosis of the primary tumor, metastasis to the regional lymph node and/or distant organs has occurred. For patients with metastatic melanoma, the current therapy is based on the use of the alkylating agent dacarbazine, and some patients also receive interleukin (IL) - 2 systemically. However, 90% of cancer deaths are caused by metastasis that are resistant to conventional therapies such as radiation and chemotherapy. There are several drugs that are available for cancer therapy; however, not a single drug emerged as a promising agent to halt these multiple steps of melanoma metastasis.
Plants have a long history of use in the treatment of cancer. Almost 60% of drugs approved for cancer treatment are of natural origin. Many experimental studies and clinical trials showed that many natural plants played an important role in blocking lung metastasis from primary tumors. Many herbal drugs such as alcoholic extract of Thuja occidentalis, aqueous-methanol (3:7) extract of Boerhaavia diffusa, methanolic extract of Withania somnifera roots, naturally occurring allyl and phenyl isothiocyanates, curcumin, and sulphorafane, etc. have been reported to inhibit melanoma metastasis.
In this study we tried to explore anti-metastatic potential of Crocus sativus L in a melanoma model. Crocus sativus commonly known as saffron which is a dietary herb of the Iridaceae family. Principal components of saffron are safranal, crocin, picrocrocin and crocetin and they are pharmacologically active. Anti-cancer and anti-tumor properties of saffron have been studied in several cancer cell lines and animal models. It is well reported that saffron can induce apoptosis in different cancer cell lines. Our previous studies show that saffron can inhibit the growth of different cancer cells such as breast, pancreatic, and lung; and it is also shown to be an active tumor remission agent in Dalton's lymphoma model. Extract of Italian Crocus sativus has been shown to be an anti-proliferative agent in B16-F10 melanoma cell line. Crocin is a major active component of saffron as reported by our group and elsewhere. Crocin possesses significant anti-proliferation effects on human colorectal cancer cells. This carotenoid can induce significant alteration of gene expression profile of T24 (transitional cell carcinoma of bladder) cells. Anti-tumor effects of Crocin are medicated at least in part by regulating the cell cycle controlling gene expression. However, efficacy of Crocin on in-vivo melanoma metastasis and its prognostic biomarkers is not yet reported. To address this issue, in this study we delineate the role of Crocin on in-vivo melanoma lung metastasis.

Saffron stigma was powdered using mortar and pestle. Powdered materials were extracted with ethanol and it is stored in -20°C until use. Active components were identified by HPLC.
Crocin was isolated from saffron as previously described. Briefly, 500 mg saffron was washed thrice with 20 mL ethyl ether, and the residual ether was evaporated in air. It was then suspended in 15 mL of 30% methanol (v/v) in distilled water and stirred for 5 minutes at room temperature. The extract was filtered through a 0.45 mm Millipore filter. It was then diluted with 10 mmol/L phosphate buffered saline (PBS, pH= 7.4), and the concentration of Crocin was adjusted to 25 mmol/L, using the coefficient €443 = 89,000 M−1 cm−1 reported for Crocin in aqueous solution. The Crocin structure was elucidated on the basis of HNMR, CNMR, IR, and mass spectral data.
B16F-10 (CRL-6475) melanoma cells were purchased from ATCC, USA. Cells were cultured in Dulbecco's modified eagle's medium (DMEM) with 10% FBS and 1% antibiotics (penicillin/streptomycin) and maintained in humidified cell incubator at 37°C and 5% CO2.
Tumor cell adhesion assay was carried out as described earlier. Briefly, B16F-10 cells were seeded on to type I collagen coated wells of flat-bottomed titer plates, in the absence and presence of Cocin (5 mg/mL, 10 mg/mL) and incubated at 37°C for 24 hours. After incubation, cells were washed with PBS and adherent cells were fixed, stained with Giemsa staining and counted under a microscope. Data were presented as mean±SD of triplicates of three independent experiments.
Tumor cell invasion assay was carried out in modified Boyden Chamber as described earlier. Briefly, the lower compartment of the chamber was filled with serum free DMEM and polycarbonate filter of 8 mm pore size was placed above this. Each filter was coated with 25 mL of type I collagen to form a thin continuous film on the top of the filter. B16F-10 melanoma cells (105/150 mL DMEM) were added to the upper chamber and incubated at 37°C in 5% CO2 for 24 hours in the presence and absence of different concentrations of Crocin (5 mg/mL, 10 mg/mL). After 24 hours of incubation, the cells on the lower surface of the membrane filter were fixed, stained and counted. Data were presented as percentage of invasion of triplicates of three independent experiments.
Tumor cell migration assay was performed similar to invasion assay except that polycarbonate filters were collagen free. Crocin (5 mg/mL, 10 mg/mL) was added along with B16F-10 melanoma cells to the upper compartment of the Boyden chamber. After incubation at 37°C for 24 hours, the number of cells migrating to the lower chamber was determined using a haemocytometer. The results are expressed as percentage motility of triplicates of three independent experiments.
The whole cell lysate was prepared from crocin (10 µg/mL) treated B16F-10 melanoma cells after 24 hours as described earlier. Then whole cell lysate were resolved in a 10% SDS polyacrylamide gel electro phoretically and electro transferred onto a nitrocellulose membrane. The immunoblots was probed with anti-E-cadherin antibody and visualized with the NBT/BCIP chromogenic substrate and documented.
Six to eight week old male C57BL/6 mice were used for the study. Mice were maintained under standardized, environmental conditions (22-28°C, 60%-70% relative humidity, 12 hours dark/light cycle and water ad libitum). All the experiments were conducted under the guidelines of Institutional Animal Ethical Committee.
Metastasis was induced to animals by injecting B16F-10 melanoma cells (1×106cells/animal) via the lateral tail vein.
Crocin was dissolved in minimum volume of ethanol and re-suspended in 1% gum acacia and was given to animals intraperitoneally (i.p) at a concentration of 250 µg and 500 mg/kg body weight.
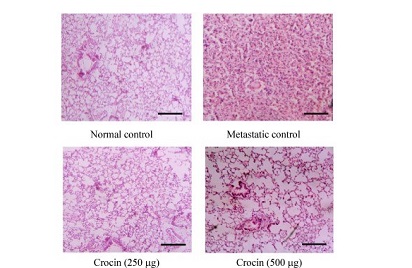
After induction of metastasis, the mice were divided in to four groups (n = 12). Group I animals were kept as normal control and group II as metastatic tumor bearing control receive saline intraperitoneally. Group III and IV received 250 µg and 500 mg/kg body weight, respectively, of crocin intraperitoneally for 10 days consecutively from the day of tumor induction. After the treatment period, six animals from each group were sacrificed and then blood was collected by heart puncture and the serum was separated. Further, lungs were excised and thoroughly washed in PBS and peripheral lung nodules were counted and used to estimate various biochemical parameters such as hydroxyproline, hexosamine, and uronic acid. Serum g-glutamyl transpeptidase (g-GT) and sialic acid were estimated by ELISA. The remaining six animals in all of the groups were observed for survival. Histopathological analysis was carried out by fixing both whole lungs, treated and untreated control animals in formaldehyde (10%) and then dehydrated using gradient alcohol and embedded in paraffin wax. Sections (4 mm) were stained with hematoxylin and eosin.
Determination of the effect of dietary Crocin on cytokine and TIMP-1 production in metastatic tumor bearing animals
Metastasis was induced in 4 groups of C57BL/6 mice (n = 12). Group I was normal control and Group II was metastatic control. Group II and III animals had received crocin at 250 µg and 500 mg/kg body weight, respectively, continuously for ten days. Six animals from each group were sacrificed on day 7 and 21 after treatment. Then serum cytokines such as IL-10, IL-6, TNF-α, IL-2 and TIMP-1 were measured using respective ELISA kits by following the manufacturer's instruction.
The animals were sacrificed on day 21 after treatment. Then lungs were excised and RNA was extracted using guanidium thiocynate and cDNA was synthesized as described elsewhere. PCR was performed using specific primers of MMP-2, MMP-9, VEGF, Erk-2, and K-ras. PCR products were resolved by agarose gel electrophoresis and visualized using ethidium bromide dye.
In-vitro data presented as mean±SD of triplicates of three independent experiments. In-vivo data were presented as mean±SD of two triplicates. Experimental data was evaluated by Students t-test and Graph PAD In stat software, Kyplot. Significant differences between each set of data were considered at the confidence level of P<0.05 and P<0.001.
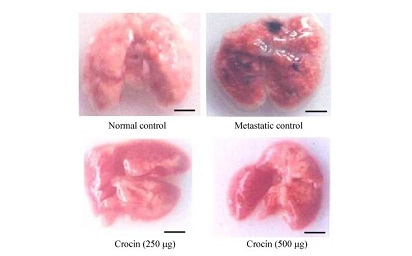
A significant reduction in the number of pulmonary metastatic colonies of B16F-10 melanoma cells were observed in Crocin treated mice compared to metastatic tumor bearing control. Administration of Crocin 250 and 500 µg/kg of bodyweight reduced percent of lung metastasis by 80% and 85% respectively in a dose dependent manner (P<0.001). Further morphology and histopathological data clearly showed significant reduction of lung metastasis in Crocin treated mice compared to untreated metastatic control. These data strongly support the anti-metastatic activity of dietary Crocin.
Mean survival time of Crocin (250 and 500 µg/kg of body weight) treated mice was extended up to 27.1 and 31 days respectively compared to untreated metastasis control mice where the survival ended after 26 days of the experimental period. Furthermore, body weight was measured in normal mice fed with Crocin (250 and 500 µg/kg of body weight) for four weeks. We found no significant difference in bodyweight of Crocin treated mice compared to control mice. This data reveals that Crocin can be tolerated by mice without eliciting toxicity.
Elevated levels of hydroxyproline (22.17±0.93 mg/mg of tissue dry weight), uronic acid (360.58±13.04 mg/100 mg tissue dry weight), and hexosamine (4.36±0.63 mg/100 mg tissue dry weight) was observed in metastatic control mice. Moreover, 250 and 500 mg/kg of body weight crocin treatment reduced their levels significantly and brought back near to normal control.
As serum sialic acid and g-GGT are lung metastatic biomarkers in this study, we measured their levels in Crocin treated metastatic mice. Both sialic acid (21.78±1.98 mg/mL) and g-GGT (118.11±5.83 nmol p-nitroaniline/mL) levels increased significantly in metastatic mice compared to normal control. Crocin treatment reduced sialic acid and g-GGT levels significantly at the end of experimental period.
B16F-10 cells treated with crocin (5 and 10 µg/mL) for 24 hours showed dose-dependent decline in adhesion (85.5% and 73.5%, respectively), invasion (57.2% and 29.2%, respectively), and migration (76.03% and 55.87%, respectively). Further, high expression of E-cadherin was observed in Crocin (10 µg/mL) treated B16F-10 cells.
As MMP2 and MMP9 facilitates the detachment of tumor cells from primary tumor site and VEGF promotes dissemination via ERK2 and Ras, TIMP1 is directly involved in inhibition of MMP's and halt metastatic process. To determine the role of Crocin on these signaling molecules in this study, we measured the serum TIMP1 level and expression of MMP's, ERK2 and Ras in crocin treated mice. Serum TIMP-1 level in untreated metastatic tumor bearing control mice was 553.96±21.41 pg/mL and increased with treatment of Crocin after 7 days (250 µg/ kg of body weight (591.71±71.33 pg/mL), 500 µg/ kg of body weight (640.20±24.09 pg/mL) and 21 days (250 µg/ kg of body weight (654.29±23.67 pg/mL), 500 µg/ kg of body weight (693.8±35.8 pg/mL). Further expression of MMP-2, MMP-9, VEGF, Erk-2 and Ras were considerably reduced by crocin treatment in comparison to metastatic control.
TNF-α (day 7: 262.12±8.42 pg/mL; day 21: 630.80±9.54 pg/mL) and IL-6 (day 7: 335.08±3.65 pg/mL; day 21: 580.28±16.6 pg/mL) were significantly elevated in metastatic mice compared to normal control. Administration of crocin at 250 and 500 µg/kg of body weight reduced TNF-α (day 7: 153.3±5.53 pg/mL and 145.45±5.3 pg/mL, respectively; day 21: 74.78±3.42 pg/mL and 65.43±2.33 pg/mL, respectively), IL-6 (day 7: 72.07±1.66 pg/mL and 68.77±3.77 pg/mL, respectively; day 21: 257.43±4.39 pg/mL and 235.89±12.2 pg/mL, respectively) levels significantly (P<0.001). In contrast, IL-2 level was reduced on day 7 (18.67±1.3 pg/mL) and slightly elevated on day 21 (630.8±9.54 pg/mL) in metastatic mice compared to control. Similarly, IL-10 level also declined on day 7 (21.23±0.95 pg/mL) and enhanced on day 21 (630.8±9.54 pg/mL) in metastatic mice compare to normal control. Treatment with crocin at 250 and 500 µg/ kg of body weight enhanced IL-2 (day 7: 30.2±0.32 pg/mL and 35.46±2.65 pg/mL, respectively; day 21: 48.71±1.26 pg/mL and 51.51±1.31 pg/mL respectively), IL-10 (day 7: 108±2.51 pg/mL and 297±5.27 pg/mL, respectively; day 21: 218±4.18 pg/mL and 312±2.78 pg/mL, respectively) levels significantly (P<0.001).
