Obesity is a major health problem in the United States and the world. Obesity and its associated metabolic disturbances, including hyperlipidemia, have been identified as risk factors for cardiovascular diseases and many other diseases.
Trans-sodium crocetinate C20H22Na2O4 is a novel synthetic carotenoid compound from Saffron that improves the diffusion of small molecules, including oxygen, in solutions. Trans-sodium crocetinate provides neuroprotection in healthy rats and rabbits. This study seeks to determine whether TSC is neuroprotective in obese mice.
MATERIALS AND METHODS
Six-week- old male mice from Charles River Laboratories (Wil- mington, MA) were fed a high-fat diet for 10 weeks before they were used in experiments.
Animal Groups and Trans-Sodium Crocetinate C20H22Na2O4 Delivery:
In the first experiment, animals were randomly divided into four groups that received normal saline (n 5 10), 0.14 mg/ kg TSC (n 5 8), 0.28 mg/kg TSC (n 5 7), or 0.7 mg/kg TSC (n 5 7). The TSC was diluted in saline to a volume of 2 ll/g body weight and given as two boluses 10 min after the onset of ischemia and reperfusion, respectively, via a tail vein.
In the second experiment, mice were randomized to receive saline (n 5 15) or 0.14 mg/kg Trans-sodium crocetinate (n 5 16) by a bolus–infusion–bolus strategy via a tail vein as described else- where (Manabe et al., 2010). Trans-sodium crocetinate was diluted in saline to a volume of 2 ll/g body weight. Ten minutes after the onset of ischemia, a bolus of one-eighth dosage of the required saline or Trans-sodium crocetinate was administered, followed by continuous infusion for 60 min to give a six-eighths dosage of saline or Trans-sodium crocetinate. At the end of the infusion, the final one-eighth dosage was injected as a bolus. These Trans-sodium crocetinate dosages were selected from (Okonkwo et al., 2003; Lapchak, 2010; Manabe et al., 2010.)
Neurological Outcome Evaluation
Li and Zuo evaluated neurological deficit scores after 3 days. Mice were then anesthetized with 5% isoflurane and perfused with normal saline through the left cardiac ventricle until clear fluid ran from the incision of the right atrium. The brain was cut into 1-mm-thick coronal slices. Each slice was observed care-fully and blindly for hemorrhagic transformation (HT), which is defined as gross blood staining in the ischemic brain tissues. An animal was considered to have HT if any of the animal’s brain slices had evidence of HT. The slices were then stained with 1% 2,3,5-triphenyltetrazolium chloride solution to evaluate edema severity and infarct volume. Edema index 5 right hemisphere volume/left hemisphere volume; corrected infarct volume in percentage 5 (left hemisphere volume – [right hemi- sphere volume – right infarction volume]) 3 100/left hemi- sphere volume. The infarct area in each brain slice was quantified in Image J (NIH, Bethesda, MD).
Brain Tissue Harvesting
For the second experiment Mice were treated with saline (n 5 7) or TSC (n 5 5) in the same way. Anesthetized with 5% isoflurane and perfused with refrigerated normal saline through the left cardiac ventricle. The brains were rapidly removed and dissected into the left and right hemispheres. The cerebral cortex, frontal cortex area 1 (Fr1), and striatum were dissected, frozen, and maintained at 280?C until use. Fr1 ipsilateral to the ischemia side is considered an ischemic penumbral region after MCAO (Zheng and Zuo, 2004; Li and Zuo, 2011
Matrix metalloproteinase (MMP) activity was assessed by gelatin zymography. Brain tissues were homogenized in phosphate-buffered saline (PBS) containing protease inhibitor cocktail and 0.005% butylated hydroxyl toluene and then in cu- bated on ice for 30 min. The homogenate was centrifuged at 13,000 rpm for 10 min at 4?C. Thirty-microliter supernatants were mixed with 23 sample buffer for gelatin zymographic analysis as described elsewhere (Wang et al., 2000). Briefly, 30 lg of proteins per lane was loaded onto 10% polyacrylamide gels containing 0.1% gelatin. After electrophoresis, the gelatino- lytic activity in the gel was revealed by incubation in a sequence in a renaturing buffer, a developing buffer, 0.5% Coomassie blue G-250 in 30% methanol and 10% acetic acid, and a destaining solution. The MMP activity was presented as clear bands at the appropriate molecular weights in the gel. Western Blotting
Approximately 30 lg proteins per lane were loaded on 10% polyacrylamide gels. After electrophoresis, proteins were transferred onto PVDF membrane that had been incubated with primary antibodies overnight at 4?C. The primary anti- bodies were rabbit polyclonal anti-MMP-9 antibody), goat polyclonal anti-tumor necrosis factor (TNF)-a antibody, rabbit polyclonal anti- interleukin (IL)-1b antibody, and mouse monoclonal anti- intercellular adhesion molecule After the membrane was washed with PBS-Tween, appropriate secondary antibodies were used. Protein bands were revealed by using the enhanced chemilumi-nescence method, photographed, and analyzed with a gel imaging system (G-Box; Syngene, Frederic, MD).
RESULTS
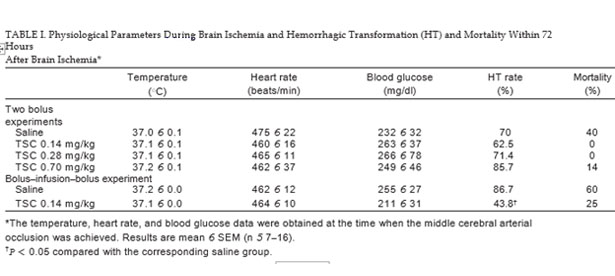
Sixteen-week-old male mice that had been fed a high-fat diet for 10 weeks were significantly heavier than the age-matched male mice that had been fed a regular diet (50.4 6 1.8 g vs. 39.8 6 0.9 g, n 5 9; P < 0.05), suggesting that the mice that had been fed the high-fat diet were obese. As previously reported, these mice also had hyperlipidemia and hyperglycemia (Deng et al., 2014). The following results were obtained with the 16-week-old male mice that had been fed a high-fat diet for 10 weeks. As shown in Table I, there were no differences in physiological parameters among the groups at the time when the MCAO was achieved
DISCUSSION
A high-fat diet was used to induce obesity in mice. This model should closely simulate human obesity because Kopelman found an excessive high-fat diet is a major cause for obesity and hyperlipidemia in humans. We found that 0.14 mg/kg Trans-sodium crocetinate C20H22Na2O4 given under a bolus– infusion–bolus regimen improved neurological outcomes after transient focal brain ischemia. Trans-sodium crocetinate has been shown to improve neurological outcomes after ischemic brain injury in young adult rats and rabbits (Lapchak, 2010; Manabe et al., 2010). This study helps finding TSC provides neuroprotection in animals with pathological conditions, such as obesity and hyperlipidemia, which often exist in patients suffering from ischemic stroke.
Gainer said Trans-sodium crocetinate C20H22Na2O4 can enhance hydrogen bonding among water molecules. This effect reduces chaos in aqueous solutions, which facilitates the diffusivity of small molecules, such as oxygen, in those solutions, including plasma and interstitial fluids. This process creates a “metabolic reflow” phenomenon in which oxygen and other small nutrients are diffused to the ischemic tissues from neighboring tissues with blood flow. Ischemic penumbral brain tissue oxygenation during transient focal brain ischemia was much improved by Trans-sodium crocetinate in rats in a previous study (Manabe et al., 2010).
Lipton said Oxidative stress-induced cell injury is an important pathophysiological process after brain ischemia and reperfusion. Trans-sodium crocetinate C20H22Na2O4 is an antioxidant, but it may require a high Trans-sodium crocetinate concentration for this effect. Nitro tyrosine-containing proteins, an indicator of oxidative stress in protein, are increased in the ischemic brain tissues. This increase in the cerebral cortex ipsilateral to the ischemic side but not in the ischemic striatum is attenuated by Trans-sodium crocetinate. The cerebral cortical tissues are a mixture of cortical tissues in the ischemic core, penumbral regions, and normal tissues. The striatum is in the ischemic core after MCAO. These results suggest that it might be difficult for Trans-sodium crocetinate to reduce the oxidative stress in the ischemic core tissues. On the other hand, HNE, an indicator for oxidative stress in lipid, was not increased in the ischemic brain tissues. TSC did not affect the HNE levels in the ischemic or no ischemic HNE tissues. These results suggest that HNE might not be a good indicator for oxidative stress in the ischemic tissues at 24 hr after brain ischemia and reperfusion.
This study shows that Trans-sodium crocetinate significantly reduces HT. HT is a complication after ischemic stroke and can worsen outcomes for patients. It also shows that Trans-sodium crocetinate reduces the MMP-9 expression and activity but not MMP-2 activity in the ischemic brain tissues. To maximize the possibility of detecting the changes in MMP activity.
The current study shows that Trans-sodium crocetinate reduces inflammation, oxidative stress, and MMP-9 expression and activity in the ischemic brain tissues, which may be the underlying biochemical changes for the Trans-sodium crocetinate induced neuroprotection, including reduced HT. However, it is not known whether these biochemical changes are the direct effects of Trans-sodium crocetinate or indirect effects from the metabolic reflow caused by Trans-sodium crocetinate. It requires mill molar levels of Trans-sodium crocetinate in solution to have significant anti-oxidative effects (Stennett et al., 2007). It would be almost impossible to reach this concentration in the ischemic brain tissues with the dose that was used (0.14 mg/kg). Thus, it is possible that Trans-sodium crocetinate, via its metabolic reflow effect, reduces the severity of brain ischemia, which leads to favorable biochemical changes.
The two-bolus regimen with three different doses did not provide neuroprotection. Given by bolus– infusion–bolus, 0.14 mg/kg Trans-sodium crocetinate was neuroprotective, but the same dose and two other higher doses are given by two boluses were not neuroprotective. The reasons for this failure to induce neuroprotection under the two-bolus regimen are not clear. A single bolus of 0.25 mg/kg Trans-sodium crocetinate within 1 hr after the brain embolization is neuroprotective in rabbits (Lapchak, 2010). Also, 0.023–0.229 mg/kg Trans-sodium crocetinate given as a bolus–infusion–bolus, started at 10 min after the onset of brain ischemia, significantly reduced brain infarct size in rats (Manabe et al., 2010). These results suggest that it is necessary to perform a dose-response study carefully to identify the dosing strategy for Trans-sodium crocetinate to be neuroprotective in conditions such as obesity and hyperlipidemia, which are often associated with stroke.
Because an obvious mechanism for Trans-sodium crocetinate to provide neuroprotection is by facilitating oxygen diffusion to the ischemic brain tissues, Trans-sodium crocetinate must be administered soon after the onset of brain ischemia.
In summary, our results suggest that 0.14 mg/kg Trans-sodium crocetinate given under the bolus–infusion–bolus regimen provides neuroprotection in obese mice. This effect may involve reduced oxidative stress, inflammation, and MMP-9 expression and activity in the ischemic brain tissues.
Share this article with others:
Rate this article or write your opinion about it:
Back to blog archive.
